Imagine if plants could pull fertilizer straight out of thin air. In a sense, that’s exactly what nitrogen-fixing bacteria do. The air around us is nearly 80% nitrogen gas, but neither plants nor animals can use nitrogen in that form. Enter these remarkable microbes – nature’s tiny chemical engineers that turn atmospheric nitrogen into forms living things can actually use. In this post, we’ll explore what nitrogen-fixing bacteria are, how they work, and look at examples of nitrogen-fixing bacteria from different environments (soil, water, symbiotic partnerships, and free-living types). By the end, you’ll see why these bacteria are key players in the nitrogen cycle and crucial for life on Earth.
What Are Nitrogen-Fixing Bacteria?
Nitrogen-fixing bacteria are prokaryotic microorganisms capable of converting nitrogen gas (N₂) from the air into “fixed” nitrogen compounds like ammonia (NH₃) that plants can use as nutrients. In other words, they make nitrogen available to living organisms by performing a process called nitrogen fixation. This is incredibly important because while nitrogen is abundant in the atmosphere, most organisms cannot use it in that gaseous form. We need nitrogen to build proteins and DNA, but plants (and the animals that eat them) can only get it once it’s been fixed into compounds like ammonia or nitrate.
Nitrogen-fixing bacteria carry out over 90% of all nitrogen fixation in nature, making them vital participants in Earth’s nitrogen cycle. Without them, the nitrogen in air would stay useless to plants, and the growth of living things would be severely limited. Thanks to these microbes, nitrogen gas is continually transformed into fertilizers in the soil, which plants use to grow. This natural fertilizer production is why legumes (like beans and peas) are protein-rich – they team up with nitrogen-fixing bacteria to get plenty of nitrogen. Farmers have long taken advantage of this by planting legumes or other cover crops as “green manure” and in crop rotations to enrich soil with organic nitrogen, reducing the need for synthetic fertilizers.
How Do These Bacteria “Fix” Nitrogen?
Turning inert nitrogen gas into a form usable by plants is no simple task. Nitrogen (N₂) is a molecule held together by one of the strongest bonds in chemistry – a triple bond. Breaking that bond requires a special enzyme and a lot of energy. Nitrogen-fixing bacteria produce an enzyme complex called nitrogenase that can split the N₂ molecule and combine it with hydrogen to form ammonia. This reaction is energy-intensive: it takes at least 16 ATP molecules of energy to fix one molecule of N₂ into two molecules of ammonia. In essence, these microbes expend a ton of energy to “crack” nitrogen gas and build fertilizer out of it.
Where does all that energy come from? Different nitrogen-fixers have different lifestyles, and they fuel nitrogenase in various ways. Free-living bacteria in the soil (which don’t partner with plants) must consume organic matter or other energy sources around them to power the process. Some of these bacteria even have the ability to use inorganic energy sources – they are chemolithotrophic nitrogen fixers that can oxidize minerals for energy. On the other hand, photosynthetic nitrogen-fixing bacteria (like many cyanobacteria) can harness sunlight and make their own sugars, which then fuel nitrogen fixation. And of course, bacteria that live in symbiosis with plants often get a direct supply of energy-rich nutrients from their plant host in exchange for the fixed nitrogen they provide.
One big challenge these bacteria face is that nitrogenase doesn’t work if oxygen is around – the enzyme is inactivated by oxygen. But many nitrogen-fixing bacteria live in soil or water where oxygen is present, or they even require oxygen for other aspects of their metabolism. So, how do they protect their nitrogenase? Nature has evolved clever solutions. Some free-living bacteria simply work anaerobically (in oxygen-free environments) or become active at night when oxygen levels are low. Others are aerobes that perform fixation in a kind of “oxygen shield” – for example, Azotobacter is an aerobic bacterium that fixes nitrogen but keeps its oxygen levels low internally by respiring very rapidly and producing protective slime layers. In symbiotic relationships, plants help create a low-oxygen safe zone: legume root nodules contain a special oxygen-binding protein called leghemoglobin (which turns the nodules pink) to tie up oxygen and protect the bacterial enzymes. Similarly, many cyanobacteria develop specialized cells called heterocysts that provide an internal anaerobic chamber for nitrogen fixation, segregating the process away from oxygen produced by photosynthesis. In short, nitrogen-fixing bacteria have found ways to keep oxygen out of the way so they can get on with the business of fixing nitrogen.
Symbiotic Nitrogen-Fixing Bacteria: Microbial Partnerships with Plants
One of the most fascinating things about nitrogen-fixing bacteria is how some of them form intimate partnerships with plants – a beautiful example of mutualism in nature. In a symbiotic relationship, the bacteria live in or on a plant’s roots and provide the plant with nitrogen, while the plant supplies the bacteria with food (carbon compounds) and shelter. Both sides benefit, making this a classic mutualistic association.
Rhizobium is a famous example of a symbiotic nitrogen-fixing bacterium. Rhizobium species are Gram-negative soil bacteria that infect the roots of legumes (plants in the pea and bean family). When a legume plant encounters its specific Rhizobium partner in the soil, a complex chemical conversation takes place. The bacteria invade the plant’s root hairs and stimulate the plant to form root nodules – little bump-like growths on the roots. Inside these nodules, millions of Rhizobium bacteria reside and begin converting N₂ from the air into ammonia, which the plant can use for growth. The plant, in turn, feeds the bacteria sugars from photosynthesis. It’s a win-win deal: the plant gets a direct nitrogen supply, and the bacteria get a comfy “home” and nutrients. This Rhizobium-legume partnership is so effective that farmers often inoculate legume seeds with the appropriate Rhizobium strain to ensure good nodule formation and robust nitrogen fixation in nitrogen-poor soils. The pink color often seen inside healthy nodules is due to leghemoglobin, the oxygen-binding protein mentioned earlier, which keeps the nodule’s environment low in oxygen for the bacteria’s benefit. Overall, Rhizobium in legume nodules is an example of symbiotic nitrogen-fixing bacteria at work – and a cornerstone of natural fertilization in agriculture.
Legumes aren’t the only plants with microbial nitrogen-fixing partners. Certain trees and shrubs (often called actinorhizal plants) form nodules with a filamentous bacterium named Frankia. Frankia is a genus of nitrogen-fixing actinomycete bacteria (related to actinomycetes like Streptomyces) that live in symbiosis with various non-legume plants, such as alder trees, bayberry, sweetgale, and seabuckthorn. Like Rhizobium, Frankia induces root nodule formation on its host plant’s roots and fixes nitrogen there. Frankia are Gram-positive bacteria (unlike Rhizobium) and they enable their host plants to thrive in nitrogen-poor soils – alders, for instance, can colonize barren land partly thanks to Frankia in their roots. These actinorhizal symbioses are especially important in certain ecosystems; for example, alder trees with Frankia often pioneer disturbed or volcanic soils, enriching them with nitrogen as they grow. This is another great example of a nitrogen-fixing mutualism: the plant provides shelter and food to the bacteria, and the bacteria provide fertilizer to the plant.
Symbiotic nitrogen-fixers aren’t limited to root nodules in terrestrial plants. A remarkable symbiosis occurs in aquatic environments between a tiny water fern called Azolla and a cyanobacterium named Anabaena azollae. Anabaena (a type of blue-green alga) lives inside cavities within the Azolla fern’s leaves and fixes nitrogen for the plant. The fern, in return, gives the cyanobacteria a safe home and nutrients. This Azolla-Anabaena symbiosis has been used in rice paddies for centuries as a natural fertilizer factory – farmers grow Azolla ferns in their flooded rice fields, and the Anabaena within them produces biofertilizer by fixing nitrogen, which boosts the rice crop’s growth when the ferns decompose. In fact, under ideal conditions Azolla with its Anabaena partner can fix an impressive amount of nitrogen (on the order of hundreds of kilograms of nitrogen per hectare per year) in a rice paddy. It’s an elegant, sustainable system: a fern and a microbe working together to pull nitrogen from thin air and fertilize the crops.
Another notable mention is Azospirillum, a genus of bacteria that doesn’t form big nodules but lives in close association with the roots of certain grasses and cereals (like corn, wheat, and rice). Azospirillum are often considered “associative” or lightly symbiotic nitrogen-fixers – they hang out in the root zone (rhizosphere) of the plant and provide some fixed nitrogen, improving plant growth. This is a looser partnership compared to the tight nodules of Rhizobium or Frankia, but it still exemplifies how plants can team up with bacteria for nitrogen. Researchers and farmers are interested in Azospirillum and similar bacteria as potential biofertilizers for crops, especially grains, to enhance yields naturally.
In all these cases – whether Rhizobium in legumes, Frankia in trees, or cyanobacteria in ferns – we see symbiotic nitrogen-fixing bacteria examples demonstrating an incredible natural mutualism. The plant-bacteria partnerships are crucial for ecosystem productivity and form the basis of many sustainable agricultural practices. 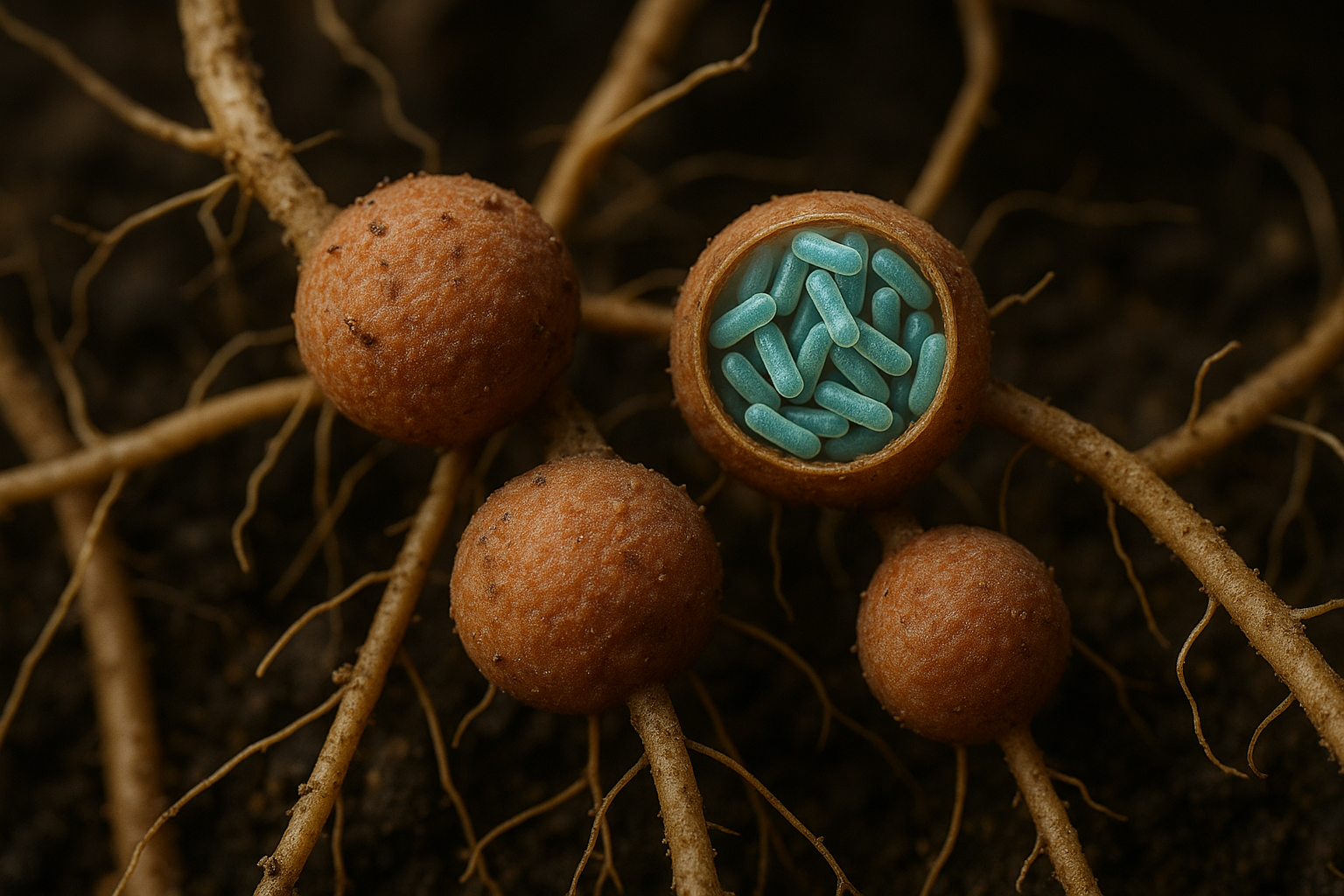
Free-Living Nitrogen-Fixing Bacteria in Soil and Water
Not all nitrogen-fixing bacteria need a plant host. Many are free-living (asymbiotic) nitrogen-fixing bacteria, meaning they roam solo in soil or water and fix nitrogen for themselves and, indirectly, for other organisms. These free-living nitrogen-fixers are found in a variety of environments – from garden soils to ponds to even the oceans. Let’s look at a few examples.
In the soil, a classic free-living nitrogen fixer is Azotobacter. Azotobacter species are Gram-negative bacteria that live in neutral to alkaline soils and even in some aquatic environments. They are aerobic (oxygen-requiring) microbes that happily fix nitrogen without any plant host, releasing ammonia into the soil as a byproduct of their metabolism. Azotobacter cells are relatively large for bacteria and have a distinctive trait: they can form thick-walled cysts to survive harsh conditions. Despite being aerobic, Azotobacter protects its nitrogenase from oxygen by respiring rapidly and possibly by producing protective pigments (some Azotobacter produce a dark pigment like a form of melanin believed to shield the enzyme from oxygen damage). They also require a lot of energy (they consume organic matter in the soil) to support the expensive fixation process. Azotobacter was one of the first aerobic free-living nitrogen fixers discovered (identified in 1901 by Martinus Beijerinck), and it remains an important organism in soil nitrogen cycling. Farmers sometimes use Azotobacter as part of biofertilizer preparations because these bacteria can boost soil fertility by increasing available nitrogen.
Another soil bacterium capable of fixing nitrogen is Clostridium. In contrast to Azotobacter, Clostridium pasteurianum and some related Clostridia are obligate anaerobes – they only grow in oxygen-free environments – and they can fix nitrogen in waterlogged soils or deep in sediments where oxygen is absent. Clostridium pasteurianum was actually the first free-living nitrogen-fixing bacterium ever discovered (by Sergey Winogradsky in the 1890s). It is a Gram-positive, spore-forming rod that lives in soil and survives by fermenting organic matter, producing things like butyric acid (it’s related to the bacteria used in fermentation processes). When oxygen is absent, Clostridium’s nitrogenase gets to work and converts N₂ to ammonia, which can then be taken up by plants once it’s released into the soil. A neat feature is that Clostridium can form dormant spores to withstand tough conditions, ensuring that when favorable anaerobic conditions return, it can resume nitrogen fixation. This makes Clostridium an important example of an anaerobic nitrogen-fixing bacteria in soils.
Besides Azotobacter and Clostridium, soils harbor other free-living nitrogen fixers too. Species of Bacillus (a Gram-positive genus) and Klebsiella (Gram-negative) are known to fix nitrogen in soil or in association with plant roots. Even some strains of Pseudomonas and Proteus bacteria have shown the ability to fix nitrogen under the right conditions. However, free-living bacteria in soil typically fix less total nitrogen than symbiotic ones, because they don’t enjoy the steady sugar diet that plant hosts provide. They often face competition and limited energy sources in the wild. Still, studies have shown they can contribute meaningfully to soil fertility – for instance, in certain farming systems with high organic matter (like crop residues left in fields), free-living nitrogen fixers can add a notable amount of nitrogen to the soil over a growing season.
Now let’s move to aquatic environments, where another set of nitrogen-fixing bacteria play a huge role. The champions here are the cyanobacteria – often referred to as blue-green algae (though they are bacteria, not true algae). Cyanobacteria are found in fresh water, oceans, and even damp soils, and many species can fix nitrogen. A well-known example is Nostoc. Nostoc is a genus of Gram-negative, photosynthetic cyanobacteria that forms gelatinous colonies and filaments. Nostoc can be free-living in moist soils, lakes, or on rocks, and it fixes nitrogen in specialized heterocyst cells (as described earlier). In fact, Nostoc often shows up as a greenish jelly-like substance on moist soil or after rains – historically sometimes called “star jelly” – which is basically a natural fertilizer blob, as Nostoc is fixing nitrogen from the air when active. Nostoc also engages in symbiotic relationships; for example, it lives in symbiosis with certain fungi to form lichens, and with plants like the small aquatic fern Azolla or even within the tissues of some liverworts and hornworts, contributing nitrogen to those partners. As a free agent, though, Nostoc in rice paddies and wetlands can significantly enrich the ecosystem with nitrogen, supporting plant growth in those environments.
Another important cyanobacterium is Anabaena (which we already met living in Azolla ferns). Anabaena can also live freely in water, forming long filamentous chains of cells with intermittent heterocysts for nitrogen fixation. In nutrient-poor lakes, Anabaena and relatives can bloom and add nitrogen to the water – though sometimes these blooms can be harmful algal blooms under certain conditions. In the oceans, filamentous cyanobacteria like Trichodesmium (sometimes called “sea sawdust”) drift in massive colonies and fix nitrogen, playing a crucial role in fertilizing the open ocean where nitrogen is often the limiting nutrient. Marine scientists have found that these cyanobacteria contribute significantly to the nitrogen budget of the oceans, supporting the growth of other plankton that form the base of the marine food web.
Whether in soil or water, free-living nitrogen-fixing bacteria examples show incredible adaptability. They might not get the cozy accommodations that symbiotic bacteria do inside plant roots, but they still manage to convert atmospheric nitrogen to a usable form, benefiting entire ecosystems. From the farmer’s field to the forest floor to the vast ocean, these unsung microbial heroes quietly keep the nitrogen cycle turning.
Why Nitrogen-Fixing Bacteria Matter in the Big Picture
As we’ve seen, without nitrogen-fixing bacteria, most plants would be starved of an essential nutrient. These microbes serve as the natural bridge between inert atmospheric nitrogen and the biologically accessible nitrogen that all organisms need. In the nitrogen cycle, nitrogen-fixing bacteria pump life into ecosystems by creating organic nitrogen compounds that feed plants. Herbivores then eat those nitrogen-rich plants, carnivores eat the herbivores, and so on – the nitrogen winds its way through the food chain thanks to its initial capture by these bacteria.
In agriculture, harnessing nitrogen-fixing bacteria is a cornerstone of sustainable farming. Farmers plant legumes like clover or soybeans not just for the crop itself but also to recharge soil nitrogen levels through their Rhizobium symbionts (this is the idea behind crop rotation and planting cover crops). Some pioneering agricultural practices involve inoculating non-legume crops with free-living or associative nitrogen fixers (like Azospirillum or engineered Rhizobia) to naturally boost yields without heavy fertilizer use. The potential benefits are huge – reduced need for synthetic nitrogen fertilizers means lower production costs and less environmental pollution. (Manufacturing synthetic ammonia fertilizer via the industrial Haber-Bosch process consumes lots of fossil fuel and has environmental side effects, whereas bacteria make ammonia at room temperature using renewable resources – talk about eco-friendly chemistry!) Overuse of chemical fertilizers has led to problems like water pollution and algal blooms, so the more we can rely on biological nitrogen fixation, the healthier our environment can be.
Ecologically, nitrogen-fixing bacteria support plant communities in some of the most challenging environments. They enable certain plants to colonize nutrient-poor soils (e.g. alder trees with Frankia in rocky terrains, or pioneer legumes in degraded lands). They also play a part in natural succession – often the first plants to grow in a barren area are those with nitrogen-fixing symbionts, preparing the way for other species by enriching the soil. In aquatic systems, cyanobacterial nitrogen fixers can alleviate nitrogen deficits and sustain productivity of phytoplankton, which in turn support fisheries and marine life.
In short, nitrogen-fixing bacteria are linchpins of both agriculture and natural ecosystems. They reduce our reliance on chemical fertilizers, improve soil health, and maintain the balance of the nitrogen cycle. Life as we know it would struggle without these microscopic fertilizer factories working behind the scenes.
Comparing Major Types of Nitrogen-Fixing Bacteria
To summarize the diversity of nitrogen fixers, here’s a brief comparison of some major types, highlighting whether they are symbiotic or free-living, their oxygen preferences, and other traits:
| Bacterium (Example) | Symbiotic or Free-Living | Oxygen Environment | Gram Stain | Typical Habitat/Host |
|---|---|---|---|---|
| Rhizobium (with legumes) | Symbiotic (mutualistic) | Aerobic (in nodules: microaerobic) | Gram-negative | Root nodules of legumes (peas, beans, clover) |
| Frankia (with actinorhizal plants) | Symbiotic (mutualistic) | Aerobic (soil; in nodules) | Gram-positive | Root nodules of certain non-legume trees (alders, etc.) |
| Azotobacter | Free-living (asymbiotic) | Strictly aerobic | Gram-negative | Soil and water; no host required |
| Clostridium pasteurianum | Free-living (asymbiotic) | Strictly anaerobic | Gram-positive | Waterlogged soils, sediments (oxygen-free zones) |
| Nostoc (a cyanobacterium) | Free-living or loose symbiosis (e.g. lichens) | Aerobic (fixes in anaerobic heterocysts) | Gram-negative | Aquatic environments, wet soils; sometimes in symbiosis (e.g. with fungi or plants) |
Table: Examples of nitrogen-fixing bacteria illustrating different lifestyles. Symbiotic types live in partnership with plants, while free-living types fix nitrogen on their own. “Aerobic” indicates they tolerate or require oxygen (often with special adaptations to protect nitrogenase), whereas “anaerobic” types operate only in oxygen-free conditions. Gram stain refers to a basic classification by cell wall structure (negative or positive).
As the table shows, nitrogen-fixing bacteria come in many forms – from free-living aerobes like Azotobacter, which wander the soil solo, to symbiotic anaerobes like Rhizobium, which find a home in plant roots. They can be Gram-negative or Gram-positive, demonstrating that this ability is spread across very different bacterial groups. Some, like Nostoc, even blur the lines by fixing nitrogen freely in the environment and forming symbioses. What unites them is their special talent: turning the inert nitrogen from the air into the nourishing ammonia that living things depend on.
Nitrogen-fixing bacteria might be microscopic, but their impact is massive. They act as living fertilizer factories, sustaining ecosystems and aiding human agriculture. Whether nestled in a root nodule or floating in the ocean, these bacteria perform the alchemy that sustains life’s protein supply chain. From a high school biology standpoint, they’re a prime example of cooperation in nature (think of the legume-Rhizobium duo as an example of mutualism) and a reminder of how interconnected life is – even our dinner plate is indirectly owed to the unseen work of soil microbes. In the grand scheme, understanding and appreciating these nitrogen-fixers can inspire us to work with natural processes (like crop rotation with legumes or using biofertilizers) for a more sustainable future. So the next time you see a clover in the field or a patch of green scum on a pond, remember the invisible bacterial partners busily turning air into fertilizer, quietly keeping the circle of life in motion.

1 thought on “Nitrogen-Fixing Bacteria – Examples and Why They’re So Important”
Comments are closed.