If you’ve ever had a bacterial infection and looked at a lab report, you might have seen the terms Gram-positive or Gram-negative bacteria. You also may have heard a doctor talk about these “Gram” types when deciding which antibiotic to prescribe. What does it all mean? The labels Gram-positive (Gram +) and Gram-negative (Gram -) distinguish two big categories of bacteria based on how their cell walls differ and how they stain in the lab. These differences turn out to be really important for how bacteria interact with our bodies and how they respond to antibiotics. Let’s break down the wall structure, the staining process, and why certain bacteria resist drugs differently depending on their Gram status – all in plain language.
The Gram Stain: Why Bacteria Turn Purple or Pink
First, a bit of history and technique: the terms Gram-positive and Gram-negative come from the Gram staining method, invented in 1884 by Danish scientist Hans Christian Gram. Gram was trying to make bacteria more visible under the microscope. In this procedure, bacteria on a slide are stained with a purple dye (crystal violet) and then treated with a series of solutions, including iodine and alcohol, and finally a counterstain (usually red/pink). Some bacteria retain the purple dye and look blue or purple under the microscope – these are called Gram-positive. Others do not hold onto the purple dye after the wash and instead take up the final red counterstain, appearing pink – these are Gram-negative.
So, in a nutshell: Gram-positive bacteria stain purple, and Gram-negative bacteria stain pink/red. But why do they behave differently in this staining process? The answer lies in their cell wall structure.
Cell Wall Structure: Thick Wall vs. Thin Wall + Outer Membrane
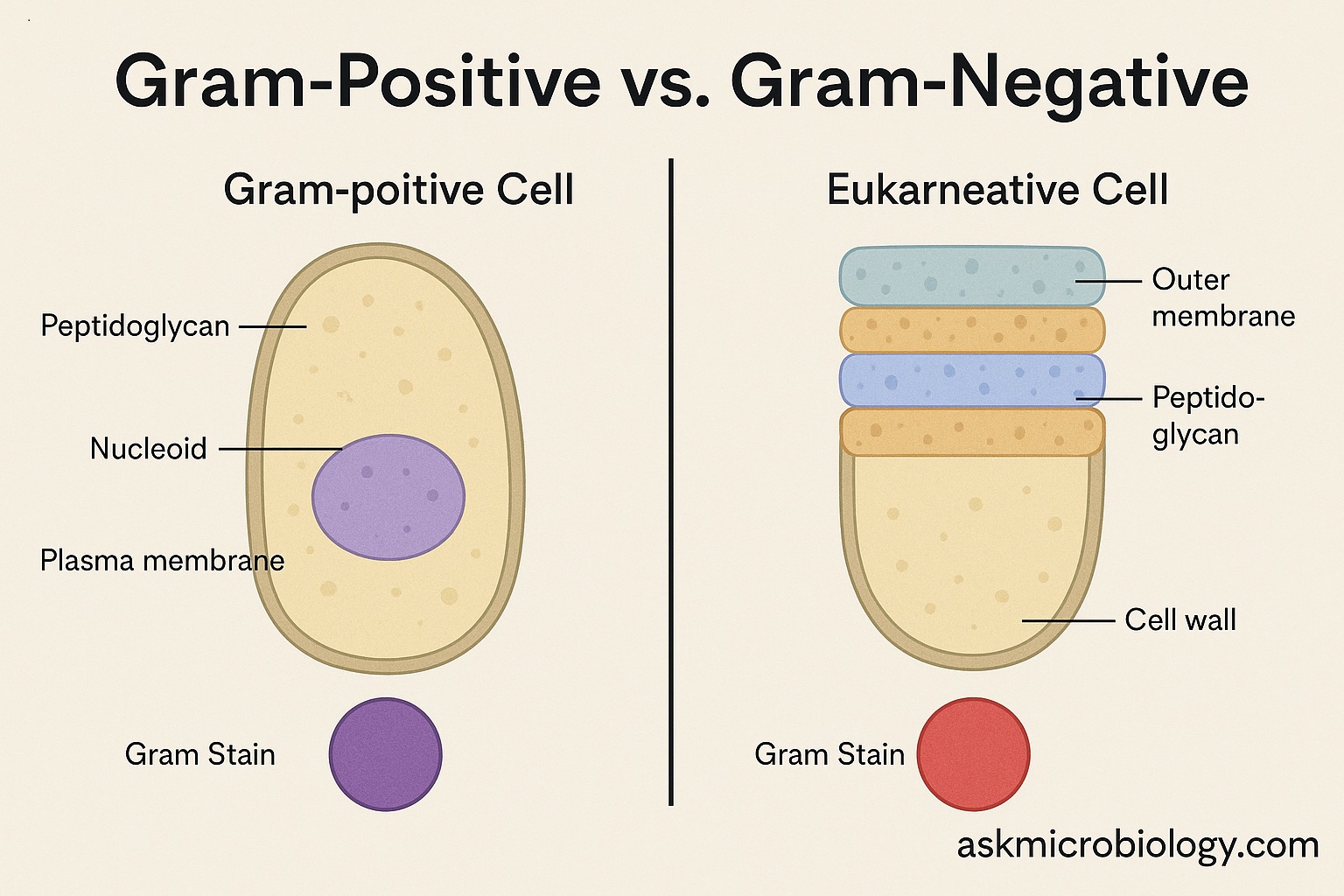
Gram-positive bacteria have a relatively thick cell wall made of peptidoglycan (a mesh-like polymer of sugars and amino acids). This thick layer traps the crystal violet-iodine complex during the Gram stain, so even when the alcohol wash is applied, the dye stays in. Imagine a thick sponge soaked in purple dye – it holds the color well. Because of this, Gram-positive cells remain purple under the microscope.
In contrast, Gram-negative bacteria have a much thinner peptidoglycan cell wall. In fact, their cell wall is sandwiched between two membranes: they have an inner cell membrane and an additional outer membrane that Gram-positive bacteria lack. The thin peptidoglycan layer in Gram-negatives cannot hold the purple dye during the alcohol wash – the dye leaches out. After that, the cells are stained by the red counterstain (like safranin), which is why they appear pink/red. If the Gram-positive cell wall is a thick sponge, the Gram-negative’s peptidoglycan is more like a thin paper – it doesn’t retain the dye strongly once washed.
To visualize it:
- Gram-positive cell: has a thick, multi-layered peptidoglycan cell wall on the outside (and beneath that, just a cell membrane). It’s kind of like a single-wall fortress – thick brick (peptidoglycan) directly exposed.
- Gram-negative cell: has a thin peptidoglycan layer but is covered by an additional outer membrane. Think of it like a thin chain-link fence (peptidoglycan) protected behind a second outer wall. The outer membrane is a lipid bilayer that contains special molecules like lipopolysaccharide (LPS), which is also known as endotoxin for reasons we’ll discuss.
Due to these structures, Gram-positive and Gram-negative bacteria also differ under the microscope in shape and arrangements often, but the Gram stain is the key differentiator.
Toxins and Effects on the Body
The structural differences lead to different types of toxins:
- Gram-positive bacteria often produce potent exotoxins – proteins they secrete into the environment. Many Gram-positive pathogens like Clostridium botulinum (which causes botulism) or Staphylococcus aureus (staph infections) secrete toxins (neurotoxins, enterotoxins, etc.) that can cause damage. The cell wall of Gram-positives also contains teichoic acids, which can trigger immune responses, but they don’t have the specific endotoxin that Gram-negatives have.
- Gram-negative bacteria have lipopolysaccharide (LPS) in their outer membrane. LPS is also known as endotoxin because when Gram-negative bacteria die and break apart, LPS can trigger strong immune reactions in the host. If a severe Gram-negative infection releases a lot of LPS into the bloodstream, it can lead to septic shock – a dangerous inflammatory response with fever, low blood pressure, and potential organ failure. This is unique to Gram-negatives because Gram-positives simply don’t have LPS in their structure. Gram-negatives can also produce exotoxins (for example, E. coli and Vibrio cholerae produce nasty toxins too), but that endotoxin LPS is a big factor in many Gram-negative infections.
In summary, Gram-positive = thick wall, primarily exotoxins; Gram-negative = thin wall + outer membrane, LPS endotoxin, plus some exotoxins too. This means the way they cause disease and how our immune system reacts can differ. For instance, infections with Gram-negative bacteria like E. coli or Pseudomonas might have a risk of endotoxin shock, whereas Gram-positive infections like strep throat (caused by Streptococcus, a Gram-positive) rely on other mechanisms of damage.
Antibiotic Response: Why Gram-Negatives Are Often Harder to Kill
Now to the part many people care about: antibiotics. The Gram status of a bacterium often influences which antibiotics will work.
- Gram-positive bacteria, with their exposed thick peptidoglycan layer, are often more susceptible to antibiotics that target cell walls. A classic example is penicillin and other β-lactam antibiotics. Penicillin’s mode of action is to interfere with peptidoglycan synthesis (it prevents bacteria from building their cell wall properly, causing them to burst). Gram-positive bacteria usually have no outer membrane to block penicillin, so penicillin can reach and weaken that thick cell wall, leading to the bacterium’s death. In fact, penicillin was historically very effective against Gram-positive infections like strep and staph. The thick porous peptidoglycan layer in Gram-positives doesn’t hinder penicillin; instead, it kind of soaks it up, and since that layer is crucial to the bacterium’s integrity, the drug works well.
- Gram-negative bacteria, on the other hand, have that outer membrane acting as a shield. This outer membrane can block many antibiotics from entering the cell. It’s like an extra defensive wall. Many Gram-negatives also have efflux pumps – proteins in their cell membrane that actively pump out harmful chemicals (including antibiotics) if they get inside. Because of these factors, Gram-negative bacteria are inherently more resistant to certain antibiotics. For example, penicillin G (the original penicillin) can’t easily penetrate Gram-negative outer membranes, so it’s not very effective against many Gram-negative species. Over the years, scientists have developed modified antibiotics (like ampicillin, amoxicillin, and others, and also entirely different classes like aminoglycosides, fluoroquinolones, etc.) that can work against Gram-negatives, but it’s generally harder.
To give a tangible example: Staphylococcus aureus (Gram-positive) vs Escherichia coli (Gram-negative). If you toss penicillin at both, the penicillin can latch onto the enzymes building the peptidoglycan in S. aureus quite readily (since it’s exposed) and kill it – at least, that was true before S. aureus developed penicillin resistance via other mechanisms like β-lactamase, but that’s another story. For E. coli, penicillin struggles to get past the outer membrane. It’s like trying to attack a castle but there’s a moat and outer wall in the way. So you might need a different approach or a modified drug that can slip through.
Another antibiotic example: vancomycin is a powerful drug that also targets the peptidoglycan cell wall. It’s great for Gram-positives (even many that resist other drugs). But vancomycin is a large molecule that cannot penetrate Gram-negative outer membranes at all, so it’s ineffective against Gram-negative bacteria. Thus, vancomycin is used for Gram-positive infections only.
The result of these structural differences is that Gram-negative bacteria are generally tougher to treat with antibiotics. In recent years, this has become a serious concern: many of the most antibiotic-resistant infections in hospitals are caused by Gram-negative bacteria. They have not only the natural outer membrane defense, but some have acquired other resistance mechanisms and can be very hard to kill. The U.S. CDC and WHO have warned about Gram-negative pathogens like Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter that are resistant to multiple drugs.
In fact, Gram-negative bacteria are such a challenge that they’re considered a top public health threat in terms of antibiotic resistance. According to the WHO, antibiotic-resistant infections (many of which are Gram-negatives) directly caused an estimated 1.27 million deaths in 2019. And a comprehensive global study in The Lancet reported a nearly 150% increase in deaths caused by certain carbapenem-resistant Gram-negative bacteria from 1990 to 2021. These statistics highlight that Gram-negatives are evolving to withstand our best drugs, partially thanks to that formidable cell envelope.
Gram-positive bacteria can also develop resistance (MRSA, for instance, is a Gram-positive Staph that’s resistant to methicillin), but we usually have more antibiotic options to throw at Gram-positives because they’re a bit more “open” to attack without an outer membrane. If one drug doesn’t work due to a specific resistance, another might. Meanwhile, a highly resistant Gram-negative might be impermeable to entire classes of antibiotics just by virtue of its outer membrane and pumps, even before specific resistance genes come into play.
Summary of Differences in a Nutshell
Let’s summarize the key differences in a quick reference format:
- Cell Wall & Structure: Gram-positive bacteria have a thick peptidoglycan cell wall and no outer membrane. Gram-negative bacteria have a thin peptidoglycan layer and an outer membrane containing lipopolysaccharide (LPS).
- Gram Stain Result: Gram-positive = purple (blue) under microscope after Gram staining. Gram-negative = pink (red) after Gram staining.
- Examples: Gram-positive genera include Staphylococcus, Streptococcus, Bacillus, Clostridium, Listeria. Gram-negative genera include Escherichia (E. coli), Salmonella, Pseudomonas, Neisseria, Haemophilus, Vibrio, etc. In infections: strep throat (Gram+), staph infections (Gram+); urinary tract infections by E. coli (Gram-), cholera by Vibrio cholerae (Gram-), gonorrhea by Neisseria gonorrhoeae (Gram-), etc.
- Toxins: Gram+ often produce exotoxins (proteins). Gram- have endotoxin (LPS in outer membrane) and also can produce exotoxins. LPS from Gram-negatives is a major factor in septic shock.
- Antibiotic Susceptibility: Gram+ usually more susceptible to drugs targeting peptidoglycan (since it’s exposed). Gram- intrinsically more resistant to certain drugs due to outer membrane barrier; often require broad-spectrum or specific antibiotics that can penetrate (or combinations with drugs that punch holes in defenses).
- Clinical Implications: Knowing the Gram status guides initial antibiotic choice. For example, if a lab report says Gram-positive cocci in clusters (likely staph), a doctor might choose a drug known to be good for Gram-positives like a cephalosporin or vancomycin (if MRSA is suspected). If it says Gram-negative rods (maybe E. coli or Pseudomonas), the doctor might opt for a different antibiotic class (like a fluoroquinolone or a carbapenem) that covers Gram-negatives. Also, infection control might be different: some Gram-negatives thrive in wet hospital environments and need special containment.
Why Does the “Gram” Difference Matter to You?
Beyond the lab and technical details, here’s why everyday folks might care about Gram-positive vs Gram-negative:
If you have an infection, the effectiveness of the treatment partly depends on this difference. For example, if you have pneumonia, the cause could be a Gram-positive bacterium (Streptococcus pneumoniae) or a Gram-negative one (Klebsiella pneumoniae). The doctor will choose antibiotics differently for these. Gram-negative pneumonia pathogens often require stronger or intravenous antibiotics. Gram-positive ones might be treated with a high-dose penicillin derivative or so on.
In a hospital setting, doctors pay attention to whether an outbreak or difficult infection is Gram-negative, because they know those can be trickier and sometimes need isolation (some Gram-negative bacteria spread in ICU environments and are very drug-resistant).
On a more general note, understanding Gram differences is a reminder of how diverse bacteria are. It’s a fundamental concept that shows evolution’s creative strategies: two structures accomplishing the same basic purpose (enclosing a cell) yet with such different consequences.
So next time you hear terms like “Gram-positive staph” or “Gram-negative rod” in a medical drama or from your doctor, you’ll know it’s about the bacteria’s armor and how it stains, which in turn affects how we fight it. It’s one of those elegant scientific distinctions that has very practical importance in medicine.
References:
- https://www.medicalnewstoday.com/articles/gram-positive-vs-gram-negative (Overview of Gram-positive vs Gram-negative differences)
- https://open.edu/openlearn/gram-positive-and-gram-negative-bacteria (Open University – cell wall and antibiotic permeability)
- https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (WHO fact sheet on antibiotic resistance)
- https://threadreaderapp.com/thread/1839155194061271107.html (Lancet study data on Gram-negative resistance increase)
- https://www.cdc.gov/gram-negative-bacteria/index.html (CDC on Gram-negative bacterial infections)