Nitrifying Bacteria—The Important Player of Nitrogen Cycle:
Nitrogen in the form of N2makes 78% of the atmosphere. It makes up the structure of living organisms. Nitrogen is present in proteins and DNA of living organisms. But despite such an abundance of nitrogen in nature, it is a limiting nutrient. Nitrogen in from of N2is useless for living creatures. So, how do living organisms get Nitrogen for their needs? How it enters in a food chain? The answer is through the nitrogen cycle that involves different bacteria. One of the crucial bacteria of the nitrogen cycle is nitrifying bacteria. In this article, we are going to look into the general aspects of nitrifying bacteria.
What are nitrifying bacteria?
By definition, nitrifying bacteria are bacteria that use inorganic compounds of nitrogen to generate energy for their living. Nitrifying bacteria are an integral part of the nitrogen cycle. After the fixation of nitrogen into ammonia by nitrogen-fixing bacteria, nitrifying bacteria play the role. Nitrifying bacteria convert the highest reduced form of nitrogen (ammonia) into the highest oxidized form, nitrate. Nitrifying bacteria are autotrophs (fix CO2into Calvin cycle). But some heterotrophic bacteria can also perform nitrification. Nitrifying bacteria, also called nitrifies, involve two types of bacteria:
Ammonia oxidizing bacteria:
These are the types of bacteria that oxidize ammonia (NH3) into nitrite NO2-. Examples include Nitrosomonas and Nitrosococcus.
Nitrite Oxidizing Bacteria:
Nitrifying bacteria that oxidize nitrite (NO2-) into nitrate (NO3-) are called nitrite oxidizers. Examples of nitrate oxidizing bacteria include Nitrobacter, Nitrococcus, Nitrospira, and Nitrospina.
Biochemistry of nitrifying Bacteria:
So the question arises, how do the nitrifying bacteria convert inorganic compounds of nitrogen (mainly ammonia) to nitrates? Many enzymes associated with nitrifiers are vital players in nitrification.
Conversion of ammonia to nitrites:
This step uses two enzymes called ammonia monooxygenase and hydroxylamine reductase. First, membrane bound monooxygenase converts ammonia into hydroxylamine. The reaction utilizes oxygen and a source of reduction. It is an endergonic reaction.
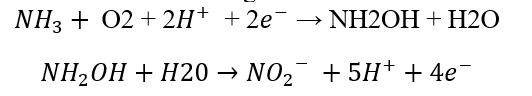
Then hydroxylamine is converted into nitrate by the enzyme hydroxylamine reductase. It utilizes water as a source of hydrogen. It is an exergonic reaction.
The redox potential for ammonia to nitrate reaction is -340mV.
Conversion of nitrites to nitrate:
The next step involves nitrite oxidoreductase that converts nitrite into nitrate. The redox potential for this reaction is 430mV.
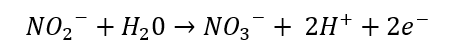
Ecology:
Nitrifiers are present mainly in soil. They are associated with the abundance of ammonia. Nitrifiers are found in lakes and rivers; there is a high content of waste in rivers and lakes, representing high levels of ammonia. They are also associated with temporal and tropical forest soil.
Why are nitrifying bacteria important?
Nitrifying bacteria keeps the soil fertile. On the contrary, denitrifying bacteria are present in the soil that transforms the nitrate into free atmospheric nitrogen, thus depleting nitrogen from the food chain. It is necessary to maintain the balance between nitrogen fixation and denitrification. Nitrifying bacteria establish an essential link between denitrification and nitrogen fixation. It maintains nitrogen losses through denitrification and leaching. It is also involved in methane (CH4) and nitrous oxide (N2O) production.
Rate of nitrification:
Nitrification rate is associated with the rate of growth of nitrifying bacteria. There are many factors upon which the rate of nitrification depends. It includes the concentration of substrate, temperature, PH, and presence of inhibitors. Here we will discuss the main factors affecting the rate of nitrification.
Temperature:
Alluvial soil has high nitrifying power. In Alluvial soil, the nitrifiers have been proved to show optimum activity at 150C and 250C. Above 340C and below 60C, the nitrifying activity started to decline.
Inhibitor:
Many inhibitors tend to stop the process of nitrification, thus slowing the nitrification rate. A prominent example is metals. Metals drastically decrease the rate of nitrification. With the presence of more than one inhibitor, an increase of inhibition is more noticeable.
PH:
Change in PH dramatically influences the rate of nitrification. The optimal PH at which nitrifying bacteria perform nitrification is 8-9.
Oxygen Concentration:
Nitrifiers are sensitive to low oxygen concentration. In an aquatic environment, the optimal reaction occurs with 0.3 to 4 milligram dissolved oxygen per liters.
APPLICATIONS OF NITRIFYING BACTERIA:
AQUARIUM:
We place our pet fishes in an aquarium and feed them. But have you ever wondered, how do they survive in the aquarium? Where does the waste material of fishes go? Ammonia is present in large amounts in the aquarium as a result of fish waste products. Accumulation of ammonia in unionized form can be a danger for fishes life. Its concentration above 0.1milligrams per liters can even cause death. Here comes the role of nitrifying bacteria. It first converts ammonia into nitrites and then into nitrate. That’s why our aquarium systems are designed in such a way to support the growth of nitrifiers. These bacteria grow on concrete surfaces in the aquarium, such as gravel, rocks, and ceramic. Nitrobacter and Nitrosomonas are generally used in the aquarium to remove nitrogen waste.
Wastewater treatment:
Wastewater consists of 99% fluid and 1% dissolved or suspended materials. Wastewater is a combination of detergents, grease, plastic, oils, and metals. It also contains an excess of nitrogen and carbon pollutants. There are many stages of wastewater treatment. One of them is a biological treatment to removes nitrogen, carbon, and organic matter from water. As the name suggests, it utilizes microorganisms, and to remove nitrogen, nitrifying bacteria play the role. Nitrifying bacteria convert ammonia into nitrates by a two-step process. But the vital point to note here is that nitrifying bacteria are not enough to remove nitrogen. Nitrates are the primary cause of Eutrophication. To remove nitrates, denitrification bacteria convert nitrates into free atmospheric oxygen. Other than denitrification, many physical processes are involved that include reverse osmosis (remove solid masses by applying pressure that allows only liquid to pass through semi-permeable membrane) and electrodialysis.
References:
- https://www.sciencedirect.com/science/article/pii/B0123485304005129
- https://www.sciencedirect.com/science/article/abs/pii/0043135480901220#:~:text=The%20maximum%20growth%20rate%20of,as%204.0%20mg%20l%E2%88%921
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3156731/
- https://www.eubios.info/TTEC/TTECPY.htm

each time i used to read smaller articles that also clear their motive, and that is also happening with this article which I am reading at this time.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Oh my goodness! Impressive article dude! Thank you so much, However I am experiencing issues with your RSS. I don’t know the reason why I am unable to subscribe to it. Is there anybody getting similar RSS problems? Anyone who knows the answer can you kindly respond? Thanks!!